
Trinitite Nuclear Glass From the Trinity Test | History, Chemistry, and Radiation
15 hours ago
6 min read
1
2
0
The Moment Uranium Left Geology and Entered History

Trinitite is not a mineral in the traditional sense, but it behaves like one in every way that matters to science. It has a composition, a structure, a radiation signature, and a formation environment that can be studied, measured, and interpreted. What separates it from every specimen that came before it is time. Not geological time, but human time.
Trinitite formed in a fraction of a second on July 16, 1945, during the first nuclear detonation at the Trinity test site in southern New Mexico. In that instant, uranium stopped being governed solely by geology and became governed by physics, engineering, and consequence.
This is uranium at the exact point it crossed that line.
The Name and the Place
The name trinitite comes directly from the Trinity test, the world’s first full-scale nuclear explosion. Early observers called the glassy residue atomsite, but the name trinitite stuck because it anchored the material to the event that created it.
The test took place in the Jornada del Muerto desert, an area composed largely of quartz sand, feldspar, and carbonate-rich soils. A plutonium implosion device was detonated atop a steel tower, instantly generating temperatures hot enough to vaporize the tower, melt the sand, and activate surrounding materials through neutron bombardment.
What fell back to Earth was trinitite.
Green trinitite is the most common form, colored by iron impurities in the desert sand. Red trinitite is rarer and formed where copper from wiring and tower components was incorporated into the melt. Both varieties represent the same process. Matter is subjected to conditions that geology cannot reproduce.
How Trinitite Forms
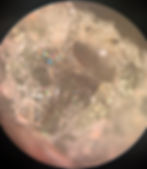
Trinitite formed through flash melting and rapid quenching. The nuclear fireball melted surface materials instantly. Molten droplets were lofted into the rising cloud, where they cooled while airborne before settling back onto the desert floor.
This cooling was chaotic. There was no time for crystal growth or chemical sorting. Quartz grains partially melted and froze mid-transition. Vesicles formed as gases escaped. Metallic inclusions were trapped inside the glass.
This is not equilibrium chemistry. This is forced chemistry.
Unlike volcanic glass, which forms from a melt that cools over minutes or hours, trinitite cooled in seconds. That difference matters. It is why trinitite has no natural equivalent.
Uranium, Plutonium, and Fallout Chemistry

Trinitite contains a complex mixture of radioactive materials. These include unfissioned plutonium from the device, uranium from both the bomb components and the surrounding environment, fission products created during the detonation, and neutron-activated elements from the soil and steel tower.
Many short-lived isotopes decayed away within days, weeks, or years. Others persisted longer. Cesium-137, strontium-90, and plutonium isotopes were once present in measurable quantities. Today, much of the detectable signal comes from longer-lived components and residual uranium.
The key point is this. Trinitite does not represent a decay chain unfolding naturally. It represents a snapshot of nuclear reactions abruptly frozen into glass.
Radiation Behavior and Modern Measurement

Measured with modern scintillation detectors such as the Radiacode, trinitite typically produces modest CPS values. This surprises new collectors. They expect something dramatic.
That expectation misses the point.

The value of trinitite lies in its spectrum, not its raw count rate. The gamma spectrum often shows a blended signature. Uranium decay products may be present, sometimes accompanied by faint cesium-137 peaks around 662 keV, depending on provenance.
This spectrum does not look like uraninite. It does not look like autunite. It does not look like any natural uranium mineral. That difference is the evidence.
Geology does not produce spectra like this.
Isolated Measurement: The Lead Castle Protocol
Open-air measurements are useful, but they are not the whole story. Trinitite in particular benefits from isolation because its signal is subtle, mixed, and easily masked by background radiation. To separate what belongs to the specimen from what belongs to the room, I also run trinitite through an isolated measurement protocol using a lead castle.
The lead castle is a stacked, offset lead enclosure built to eliminate direct line of sight gaps. The Radiacode detector and the specimen are placed together inside the box, fully enclosed on all sides except the removable access opening. This configuration dramatically suppresses ambient gamma background from building materials, cosmic radiation, and nearby specimens.
Before any specimen is introduced, a full background spectrum is recorded inside the closed lead castle. This establishes a true zero context for the enclosure itself. The result is a spectrum that is markedly quieter than open air, with background features collapsed and smoothed.
Lead Castle Background Spectrum

This background spectrum is intentionally boring. That is the goal. When the room disappears, whatever remains belongs to the specimen.
Once the baseline is saved, the trinitite is placed inside the lead castle directly beneath the detector, maintaining fixed geometry. The detector remains in the same orientation and distance relative to the sample. The enclosure is closed and the acquisition begins.
Lead Castle Trinitite Spectrum

Compared to the open-air spectrum, several things become immediately clear. Background noise is suppressed. Low-intensity features become easier to resolve. The blended uranium signature sharpens, and faint peaks that would otherwise be lost become visible.
This is not about increasing CPS. In fact, total counts often decrease. What improves is signal clarity. The lead castle does not make the specimen stronger. It makes the data cleaner.
For trinitite, this matters. The material does not behave like a natural uranium mineral, and its spectrum reflects artificial isotope mixing and rapid formation. Isolation allows those differences to stand on their own instead of being drowned out by environmental radiation.
This protocol will appear throughout future articles when subtle spectral differences matter. Open-air spectra show how a specimen behaves in the real world. Lead castle spectra show what the specimen is doing on its own. Both are honest. Together, they tell the full story.
Why Trinitite Matters Scientifically

Trinitite is a material archive. It records extreme temperature, neutron flux, and rapid quenching in a single object. It allows scientists to study how elements partition under conditions far outside natural systems.
It challenges definitions. Is it a mineral. A glass. A rock. A nuclear artifact. The answer is yes.
For students, trinitite demonstrates the difference between radioactive minerals and nuclear materials. For geochemists, it provides a reference point for artificial isotopic mixing. For historians of science, it is physical proof of the moment the nuclear age began.
This is not novelty glass. It is data.
Legal and Ethical Context

Collection of trinitite from the Trinity Site itself is illegal. All legally held specimens were collected decades ago, before protections were enacted, or originate from private land outside the restricted area where fallout glass was deposited.
Responsible collectors rely on legacy material with documented provenance. This matters. Trinitite is not just radioactive. It is historically radioactive.
Owning it carries responsibility.
How Trinitite Fits Into a Uranium Collection
Trinitite occupies a position no natural uranium mineral can. It is not part of the uranium alteration sequence. It interrupts it.
Uraninite, soddyite, zippeite, and shrockingerite record uranium responding to water, oxygen, and time. Trinitite records uranium responding to heat, pressure, and physics beyond geology.
In a display, it becomes the control sample. The moment everything changes.
After trinitite, every uranium mineral feels different.
Where the Story Continues
Specimens curated at RadioactiveRock.com are selected to document uranium behavior across its full spectrum, from dense primary ores to fragile surface minerals and historically significant materials like trinitite. Each piece is photographed, measured, and documented using repeatable methods so collectors understand not just what they own, but what it represents.
Up Next
Next, I will return to natural systems with fresh context, revisiting uranium minerals that altered slowly, patiently, and without a fireball. After trinitite, the quiet chemistry speaks louder.
Curite, Soddyite, and Meta-Torbernite, a uranium assemblage that refuses to sit quietly. From the Katanga Copper Belt of the Democratic Republic of Congo, this specimen captures soddyite, curite, and meta-torbernite locked together in a single alteration sequence, showing uranium actively migrating from U–Si oxides into Pb–U oxides and finally into Cu–U phosphates.
Curite takes its name from Marie Curie, not as a romantic gesture, but as a mineralogical warning sign. It forms only after uranium has already altered once and remains chemically aggressive, radiogenic, and mobile. This is not a trophy crystal or a static endpoint. It is uranium mid-conversation with fluids, oxygen, copper, and time. Every color shift and texture change marks a chemical decision frozen just long enough to be studied. If you want to understand how uranium actually behaves in a real system instead of how it looks in a cabinet, this assemblage is where the story gets honest.
Stay curious, stay safe, and keep your detectors chirping.





